-
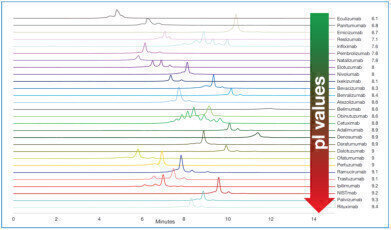 Fig: Separation of 28 different commercial Mabs using YMC’s Bio Pro IEX SF column
Fig: Separation of 28 different commercial Mabs using YMC’s Bio Pro IEX SF column
Bioanalytical
MS compatible charge variant analysis of 28 commercial monoclonal antibodies by CEX
Jan 26 2021
Cation exchange chromatography (CEX) is perceived to be the gold standard for the charge sensitive characterisation of monoclonal antibodies (MAbs). Acidic and basic variants caused by chemical or enzymatic modifications can be separated from the main isoform of the MAb. These antibody variants have to be critically evaluated since differences in impurities and/or degradation products can lead to severe undesirable side effects.
Analysis of 28 MAbs with a broad range of pI
The application note described here is based on data produced by the University of Geneva, School of Pharmaceutical Sciences according to the method described by Yan et al. [1]. It shows the analysis of 28 commercial MAbs with different isoelectric points (pI 6.1–9.4) using YMC’s BioPro IEX SF column. Each MAb could be separated from acidic and basic variants which can be further characterised if coupled to a mass spectrometer (using a possible setup as described by Yan et al.). In contrast to the publication by Yan, fluorescence detection was used in this application note.
To achieve an acceptable elution window the initial and final ratio of mobile phase B was tuned for each MAb depending on its isoelectric point starting with an initial ratio of 20–30 % B (higher ratio for MAbs with higher pI).
Direct coupling of SCX to ultrasensitive MS
In a former application note based on a study by Yan et al., high resolution SCX (strong cation exchange chromatography) was coupled directly to an ultra sensitive online native MS [1]. A combined pH and salt gradient based on a MS compatible buffer system was utilised. YMC’s BioPro IEX SF, a non-porous strong cation exchange column, was used.
Reliable – Robust – Reproducible: BioPro IEX by YMC
One of the crucial requirements for any chromatographic QC method is a reliable lot-to-lot reproducibility for the column used. Unlike many other IEX columns on the market including widely used “standard” ones, YMC’s BioPro IEX columns provide excellent lot-to-lot reproducibility together with superior resolution. These features make them a first choice for Bio QC!
BioPro IEX columns are specifically designed for separation of antibodies, proteins, peptides, nucleic acids and oligonucleotides showing higher binding capacity and recovery of biomolecules compared to conventional IEX columns.
[1] Anal. Chem., 2018, 90, 13013-20.
Events
Jan 20 2025 Amsterdam, Netherlands
Feb 03 2025 Dubai, UAE
Feb 05 2025 Guangzhou, China
Mar 01 2025 Boston, MA, USA
Mar 04 2025 Berlin, Germany













