-
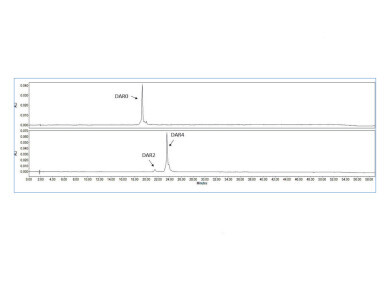 Figure 2: Analysis of a site-specific ADC before (top) and after (bottom) drug conjugation.
Figure 2: Analysis of a site-specific ADC before (top) and after (bottom) drug conjugation. -
 Figure 1: DAR determination of a 3rd generation, site-specific ADC using reversed phase chromatography
Figure 1: DAR determination of a 3rd generation, site-specific ADC using reversed phase chromatography
Columns (LC)
DAR determination of a 3rd generation, site-specific ADC using reversed phase chromatography
Dec 10 2024
Antibody-drug conjugates (ADCs) represent a rapidly evolving class of targeted therapies, with three distinct generations of conjugation methods. The first generation utilises lysine residues for drug attachment, while the second generation relies on cysteine coupling. The third generation, however, marks a significant advancement with site-specific conjugation, where cytotoxic drugs are attached at defined positions on the antibody, leading to a more homogeneous ADC with a precise drug-to-antibody ratio (DAR) of either 2 or 4.
While hydrophobic interaction chromatography (HIC) has traditionally been used for DAR determination, this approach may not offer the optimal resolution for third-generation ADCs. These molecules exhibit lower hydrophobicity compared to their first- and second-generation counterparts. In contrast, reversed phase chromatography (RP) provides enhanced resolution for these less hydrophobic ADCs, as the stronger interactions with the hydrophobic RP stationary phase become advantageous. Moreover, RP facilitates the integration of mass spectrometry detection, offering a streamlined workflow compared to the high salt concentrations typically required in HIC.
This application note demonstrates the accurate DAR determination of a site-specific ADC using a YMC-Triart Bio C4 column, a robust wide-pore hybrid silica-based column designed to deliver reliable, high-resolution separations.
Figure 2 demonstrates that the drug conjugation is complete, as no bare antibody can be detected. The majority of the payload has a DAR of 4, and the ADC with a DAR of 2 can be separated from the main peak with high resolution.
*Application data by courtesy of KriSan Biotech Co., Ltd.
You can find more ADC applications in the YMC Application Collection for Antibodies and ADCs.
Events
May 11 2025 Vienna, Austria
May 18 2025 Tempe. AZ, USA
May 21 2025 Birmingham, UK
Jun 01 2025 Baltimore, MD, USA
Jun 15 2025 Bruges, Belgium















