Purification
Next generation RP phase for enhanced insulin purification
Apr 25 2023
For the purification of insulin, YMC has specifically developed a new reversed phase material that addresses the specific needs of insulin purifications which results in a significant improvement for every production process: The development of YMC-Triart Prep Bio200 C8 was carried out in cooperation with two independent insulin manufacturers.
This application note shows real-life data obtained from insulin manufacturing. These examples demonstrate the great potential for optimisation of existing insulin processes. The performance of this phase exceeded the expectations of the cooperating partners. In fact, the loading can be up to 100% greater whilst still reaching the set purity of 99.5!
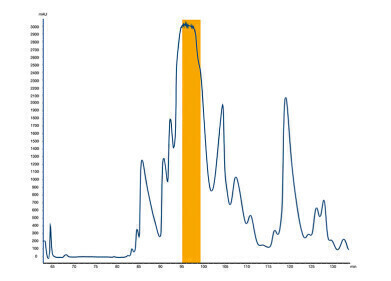
Example 1: Company A
The target values for the purity and the recovery were set by the cooperation partner from company A. The purity shall be minimum 99.5%, the recovery shall be higher than 80%. The crude sample had a purity of 94.9%, and the loading was defined with 30 mg/g stationary phase. The set acceptance criteria were significantly exceeded. Instead of reaching a recovery of 80% (set as minimum), a recovery of 93% was achieved!
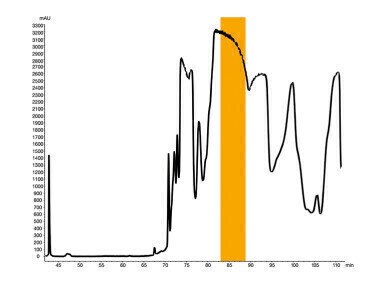
Example 2: Company B
The target values for the purity and the recovery were set by the second cooperation partner from company B. The purity shall be minimum 99.5%, the recovery shall be again higher than 80%. The crude sample had a purity of 75.8%, and the loading was defined with 26.5 mg/g stationary phase. By using the existing method parameters, no fraction was found with the required purity of 99.5%. In order to improve the separation and consequently the purity of the fractions, a salt was added to the mobile phase. With the salt added, the results were significantly improved. The set criteria were again exceeded in terms of purity and recovery. As a results, this process allows a purity of 99.7% based on a crude with a purity of 75.8%. The recovery reached a level of 87%!
Example 2: Comparison data
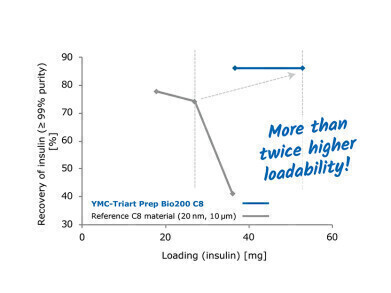
The Example 2 was also tested with a currently available stationary phase with comparable phase parameters (200 Å pore size, 10 µm particle size, C8 modification). This demonstrated that YMC-Triart Prep Bio200 C8 allows more than 100% higher loading to be achieved with the same purity. Therefore the productivity of the process can be doubled!
This developed phase for insulin processes, YMC-Triart Prep Bio200 C8, allows the greatest productivity for all insulin processes. Based on the trusted cooperation with insulin manufacturers, it was possible to design a completely new stationary phase with superior properties. The examples shown demonstrate the ability of this phase to set new benchmarks for insulin purifications.
Events
May 11 2025 Vienna, Austria
May 18 2025 Tempe. AZ, USA
May 21 2025 Birmingham, UK
Jun 01 2025 Baltimore, MD, USA
Jun 15 2025 Bruges, Belgium