-
 Figure 3: Extracted ion chromatograms of the antisense and sense strands of lumasiran (Lum_AS and Lum_S) in the reconstituted plasma at the LLOQ [1].
Figure 3: Extracted ion chromatograms of the antisense and sense strands of lumasiran (Lum_AS and Lum_S) in the reconstituted plasma at the LLOQ [1]. -
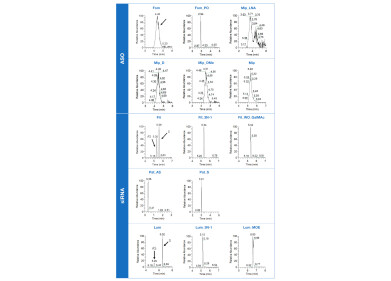
 Figure 1: RP-MS analysis of therapeutic oligonucleotides without the use of ion pair reagents
Figure 1: RP-MS analysis of therapeutic oligonucleotides without the use of ion pair reagents
Liquid chromatography
RP-MS analysis of therapeutic oligonucleotides without the use of ion pair reagents
Oct 22 2024
Ion pair reversed phase liquid chromatography (IP-RP) is the gold standard for the analysis of oligonucleotides. However, the costs and the environmental aspects of commonly used fluor alcohols like 1,1,1,3,3,3-hexafluoro-2-propanol (HFIP), require alternative approaches.
This application note based on the work of Hayashi and Sun [1] shows a non-IP-RP method for the analysis of therapeutic oligonucleotides using ammonium bicarbonate as mobile phase additive. Ammonium bicarbonate seems to have a positive effect on the MS sensitivity. Under heated conditions (about 60 °C), it degrades into ammonia and highly volatile CO2 forming bubbles in ESI droplets [2]. Furthermore, the equilibrium between ammonia and ammonium ions could be an important factor (NH4+ ⇌ NH3+ H+). In an electrospray ionisation (ESI) droplet the ammonia vaporises leaving protons in the droplet, that can form adducts with the analytes. Therefore, ammonium bicarbonate in combination with the high performance of the used bioinert coated YMC Accura Triart C18 column are the ideal prerequisites for a highly sensitive detection by mass spectrometry (MS).
The method’s applicability is proved by analysing various commercially available oligonucleotides with chemical modifications including six antisense oligonucleotides (ASO), three siRNA as well as four of their analogues. The analysis of lumasiran (Lum, Oxlumo®) in reconstituted plasma demonstrates the applicability of this method for bioanalyses.
All oligonucleotides can be detected using the ammonium bicarbonate-based mobile phase. However, the retention time depends on the oligonucleotide and the ammonium bicarbonate concentration. For example, the antisense strand of patisiran (Onpattro®) elutes too close to the void volume using 10 mM ammonium bicarbonate (not shown), but the retention time is almost three times longer with 20 mM ammonium bicarbonate [1].
Figure 2 presents the extracted ion chromatograms of the evaluated oligonucleotides. The oligonucleotides show sharp peakes, except for the phosphorotioated oligonucleotides like fomivirsen (Fom, Vitravene®) and mipomersen (Mip, Kynamro®) exhibiting much broader peaks. This is likely due to the presence of various stereoisomers.
To prove the applicability to bioanalyses the antisense and sense strands of lumasiran (Lum_AS and Lum_S) are used as model oligonucleotides (see Figure 3). The analysis of the blank sample does not show any interfering peaks at the relevant retention times.
The lower limit of quantification (LLOQ) is 1 ng/mL for Lum_AS and 0.5 ng/mL for Lum_S, respectively. The findings of this study indicate that this ammonium bicarbonate-based RP method is suitable for the bioanalysis of therapeutic oligonucleotides.
Discover more helpful oligonucleotide applications in the YMC Oligonucleotide Columns brochure.
References:
[1] Yoshiharu Hayashi and Yuchen Sun, Journal of the American Society for Mass Spectrometry Article ASAP, DOI: 10.1021/jasms.4c00270
[2] House, J. E. Decomposition of ammonium carbonate and ammonium bicarbonate and proton affinities of the anions. Inorganic and Nuclear Chemistry Letters 1980, 16 (4), 185−187.
Events
May 11 2025 Vienna, Austria
May 18 2025 Tempe. AZ, USA
May 21 2025 Birmingham, UK
Jun 01 2025 Baltimore, MD, USA
Jun 15 2025 Bruges, Belgium