-
 Bioinert YMC-Triart metal-free columns for excellent peak shapes and high sensitivities.
Bioinert YMC-Triart metal-free columns for excellent peak shapes and high sensitivities. -
 Oligonucleotide analysis using a bioinert YMC-Triart C8 metal-free column.
Oligonucleotide analysis using a bioinert YMC-Triart C8 metal-free column.
LC-MS
LC-HRMS analysis of the antisense oligonucleotide Mipomersen (Kynamro®)
Mar 23 2021
Antisense oligonucleotides (ASOs) have an outstanding therapeutic potential, since they are used for the therapy of genetic diseases. Due to the specific binding of an ASO to the complementary sequence of the RNA of a target protein, protein expression can be suppressed.
Recent approvals of commercial ASOs
Recently, gapmer ASOs with increased in vivo stability have been introduced. They are chimeric RNA/DNA hybrids containing modified RNA nucleotides at the flanks and DNA nucleotides at the central block. Several gapmer ASOs have been clinically approved in 2020, including inotersen (Tegsedi®), volanesorsen (Waylivra®) and mipomersen (Kynamro®). These gapmer ASOs typically comprise phosphorothioate (PS) linkers and 2’-O-methoxyethyl (2’-MEO) modifications.
Mipomersen, which is serving as the model compound here, was developed to treat homozygous familial hypercholesterolemia. It inhibits the production of the low-density lipoprotein (LDL) and very low-density lipoprotein (VLDL) and therefore reduces their concentration in blood.
Bioanalytical methods required
Despite their promising therapeutic potential, bioanalytical methods for ASOs have not been well developed and require further optimisation. Also fully validated LC-HRMS methods have not been established yet. Y. Sun et al. have recently reported a successful development of a sensitive LC-HRMS method for mipomersen in rat plasma as a model compound for 2’-MOE gapmers [1].
LC-HRMS method using bioinert YMC column
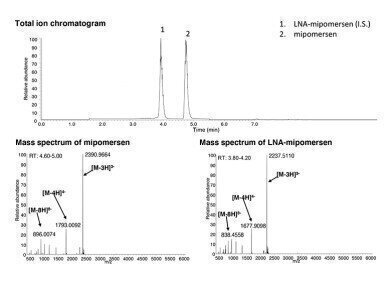
In this application note based on the study by Y. Sun et al, a bioinert YMC-Triart C8 metal-free UHPLC column was used. In addition, versatile YMC-Triart C18 and the very hydrophobic YMC-Triart C18 ExRS (both also in metal-free column hardware) were also tested, but YMC-Triart C8 demonstrated the best separation of mipomersen and LNA-mipomersen as internal standard. However, the use of a bioinert column turned out to be crucial, as the use of a standard C18 column in stainless steel hardware showed severe carry-over.
Optimised conditions
TEA/HFIP (triethylamine/1,1,1,3,3,3-hexafluoro-2-propanol) were used as the ion pairing reagents. Their concentrations were optimised to improve the separation, the peak shapes, avoid carry-over and ensure high reproducibility. The final concentrations are 28.0 mM TEA and 135.8 mM HFIP. In addition, it was essential that the column was prewashed prior to the first use with 0.1% phosphoric acid in water/methanol (30/70) for 1 h to prevent non-specific binding of ASOs to the column. The resulting method not only showed potential for measuring preclinical samples of very low ASO concentrations, but also for the future analyses of ASOs of the gapmer type.
[1] Y. Sun, Bioanalysis,2020-0225
Events
May 18 2025 Tempe. AZ, USA
May 21 2025 Birmingham, UK
Jun 01 2025 Baltimore, MD, USA
Jun 15 2025 Bruges, Belgium
Jul 14 2025 Kuala Lumpur, Malaylsia