-
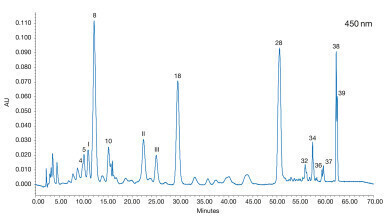 Figure 3: Analysis of a human plasma sample 24 h after the consumption of a mix of vegetables and fruits [1]
Figure 3: Analysis of a human plasma sample 24 h after the consumption of a mix of vegetables and fruits [1] -
 Figure 1: Successfully separating complex mixtures of carotenoids.
Figure 1: Successfully separating complex mixtures of carotenoids.
HPLC, UHPLC
Separating complex carotenoid mixtures
Dec 13 2023
Carotenoids tend to form geometric isomers, so they can occur as all-trans (all-E) or as cis (Z) isomers. They arise, for example, from the effects of light or heat on vegetables and fruits or their extracts. They also occur naturally or are formed in the human body. Due to the different shape of the Z isomers (kinked shape instead of linear and rigid), the isomers can differ drastically in their properties such as solubilisation or adsorption as well as transport in the human body. Lycopene, for example, occurs predominantly in foods as the all-E isomer, while in the human body it predominantly occurs as the Z isomer. This means that the lycopene is either immediately isomerised by the human body or the all-E form is poorly absorbed.
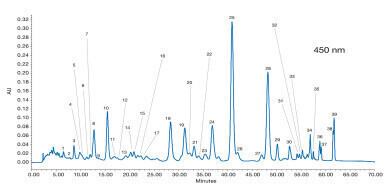
Therefore, appropriate analytical methods for detailed profiling of carotenoid isomers in nutritional studies are of great importance. This application note demonstrates the separation of up to 48 carotenoids by detection at different wavelengths using a reliable YMC Carotenoid column with C30 modification [1].
Standards of lutein, zeaxanthin, β-cryptoxanthin, α-carotene, β-carotene, lycopene, phytoene and phytofluene dissolved in ethanol were heated at 80 °C for 30 min to achieve stereomutation. The isomers of phytoene and phytofluene were detected at 285 and 347 nm. A mixture of the other extracts was used to optimise the separation with a detection at 450 nm (see Figure 2). Refer to Table 2 of the Application Note for peak assignments.
With this method a human plasma sample 24 h after the consumption of carotenoid containing vegetables and fruits (carrot, celery, beets, parsley, lettuce, watercress, spinach and tangerine) was analysed. Several carotenoids were found (see Figure 3). Phytoene was not detected. Furthermore, three unidentified peaks (I – III) were detected, which do not match with the carotenoid isomers previously studied. These can be allocated to 3’-epilutein (I) and 3’-dehydrolutein (II,III) due to their absorption maxima. These substances have been detected in human serum and / or human retina in previous studies [2,3].
Visit the Expertise Portal from YMC for even more valuable applications, technical documents and beneficial Expert Tips.
Literature
[1] A. J. Melendez-Martinez, C.M. Stinco, C. Liu, X.-D. Wang. A simple HPLC method for the comprehensive analysis of cis/trans (Z/E) geometrical isomers of carotenoids for nutritional studies, Food Chem. 138 (2013)1341-1350
[2] G. Britton, S. Liaaen-Jensen, H. Pfander, Carotenoids. Handbook (2004), Switzerland: Birkhäuser.
[3] W. Schalch, J.T. Landrum, R.A. Bone, Nutrition and health (2009), Vol. 5, pp. 301–334
Events
May 11 2025 Vienna, Austria
May 18 2025 Tempe. AZ, USA
May 21 2025 Birmingham, UK
Jun 01 2025 Baltimore, MD, USA
Jun 15 2025 Bruges, Belgium