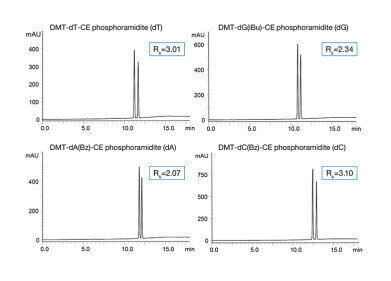
-
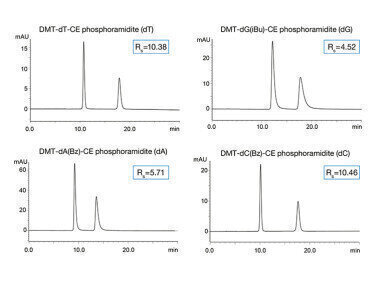 Fig. 2: Separation of four phosphoramidites using RP conditions.
Fig. 2: Separation of four phosphoramidites using RP conditions. -
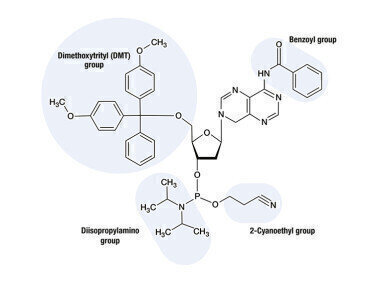 Fig 1: Protecting groups of an adenosine based phosphoramidite.
Fig 1: Protecting groups of an adenosine based phosphoramidite.
HPLC, UHPLC
HPLC Analysis of Phosphoramidites using RP or NP conditions - Maximum Flexibility with YMC-Triart
Apr 26 2022
Synthetic oligonucleotides are extremely promising candidates for biopharmaceuticals in a wide range of diseases. This is why nucleic acid therapeutics such as silencing RNA (siRNA), messenger RNA (mRNA) and antisense oligonucleotides (ASOs) are gaining more and more attention in current research.
Phosphoramidites essential for synthesis of oligonucleotides
Phosphoramidites are the essential part of the chemical synthesis of oligonucleotides, short fragments of nucleotides and analogues. To prevent any side reactions on residual reactive sites such as hydroxyl and amino groups during oligonucleotide synthesis, these groups need to be protected. Therefore, the highly reactive native nucleotides are modified with four different protecting groups.
Benzoyl (Bz) or isobutyryl (iBu) groups protect the amino group of the base. This is only required for the nucleobases adenine, guanine and cytosine as they all have primary amino groups which otherwise would attach to another nucleotide. For thymine, this protecting group is not required since it only has a less reactive secondary amine group. The 5’-hydroxyl group of the sugar moiety is protected by a dimethoxytrityl (DMT) group. Inhibiting side reactions at the 3’-hydroxyl group of the sugar moiety is performed by two groups: a diisopropylamino and a 2-cyanoethyl (CE) group are attached to the phosphate atom. The first figure shows the protecting groups using the example of an adenosine based phosphoramidite.
Analysis in RP or NP mode using YMC-Triart hybrid silica columns
The chemical oligonucleotide synthesis is performed in four major steps: detritylation of the phosphoramidite, coupling, oxidation and capping. During this process, errors can occur which is why the purity of the phosphoramidites needs to be closely monitored. In this application note two different separation modes – reversed phase (RP) and normal phase (NP) – are used for the analysis of four different phosphoramidites. Due to the complexity of these molecules different interactions can be used in order to achieve reasonable retention and resolution. As phosphoramidites have a chiral centre at the phosphorus atom two isomers are present resulting in double peaks visualised in the chromatograms.
All separations were performed using the highly robust YMC-Triart columns which are an ideal choice for modern biochromatography applications. For RP separations a YMC-Triart C18 column was used. By applying a gradient up to 95%B and only moderate temperatures sharp peaks are obtained. Due to the special multi-stage endcapping process for YMC-Triart C18, no tailing is observed.
Besides employing the hydrophobic interactions of RP chromatography, the separation of phosphoramidites is also possible by focussing on the polar interactions using NP conditions. A YMC-Triart SIL column was used which is based on bare hybrid silica.
Although the substances have several basic parts in their structure, there is no tailing observed for the phosphoramidites based on thymine and cytosine using a mobile phase containing triethylamine as additive. Excellent resolution was obtained for all compounds.
Events
May 11 2025 Vienna, Austria
May 18 2025 Tempe. AZ, USA
May 21 2025 Birmingham, UK
Jun 01 2025 Baltimore, MD, USA
Jun 15 2025 Bruges, Belgium