Columns (LC)
Successful Cleaning and Cleaning Validation of Glass Columns
May 24 2022
For pharmaceutical and biopharmaceutical LC-processes an efficient, validated cleaning method is essential. The optimum cleaning procedure
- reliably removes impurities,
- is fast to avoid long down times
- is compatible with resin and equipment.
During cleaning validation these points need to be evaluated in order to guarantee product safety and to fulfil regulatory requirements.
The most time-efficient cleaning procedure
The most time-efficient cleaning procedure is achieved with a packed and assembled column. Cleaning-in-place (CIP) is the preferred method for preparative chromatography. A robust and efficient CIP procedure ensures product integrity and increased process lifetime.
But how can column design help to establish an efficient cleaning-in-place method? The most important requirement for the column technology is an efficient distribution of the cleaning agent through the column in combination with reduced dead areas. In addition, easy to clean surfaces and frits influence the versatility of a column for CIP procedures.
In a recent in-house study, the influence of the column hardware on cleaning efficiency was investigated. The results show the excellent flow distribution of YMC glass columns allowing reliable, fast and efficient CIP procedures.
The importance of the column hardware
To demonstrate the importance of the most appropriate column hardware for high cleaning efficiency in CIP, a cleaning study was performed with two glass columns in different scales: a laboratory scale ECO glass column with an inner diameter of 25 mm and a pilot/production scale YMC Pilot glass column with an inner diameter of 140 mm. Both column dimensions were used for stepwise scale-up procedure with different kind of resins.
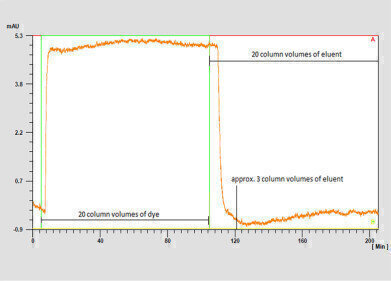
Detailed experiments were carried out in which the dye Coomassie Brilliant Blue G-250 was dissolved in 80% isopropanol and injected on the column. The dye was subsequently rinsed with the same concentration of isopropanol. This whole process was monitored by UV detection.
The complete removal of the dye was achieved after washing with 3 CV of the eluent as shown by the signal returning to the baseline.
The final inspection of the column frits after column disassembly showed no visible signs of dye residue on the frits after the method had been carried out.
Excellent cleaning efficiency with YMC glass columns
This cleaning validation shows that a successful CIP process can be carried out in laboratory and pilot scale. YMC glass columns are equipped with fully porous frits to ensure an optimal distribution of cleaning agents. This particular column design
- reduces production downtimes,
- enables product safety in one step,
- is applicable to all CIP solvents.
To see the method parameters and the complete study results please read the full Whitepaper.
Events
Apr 22 2025 Kintex, South Korea
Analytica Anacon India & IndiaLabExpo
Apr 23 2025 Mumbai, India
Apr 27 2025 Portland, OR, USA
May 11 2025 Vienna, Austria
May 18 2025 Tempe. AZ, USA