Columns (LC)
LC/MS Analysis of Pain Management Opioids with Ascentis® Express Biphenyl HPLC Columns
Nov 23 2016
The improvement of pain management therapies is a persisting clinical need. Determination of benefits and side effects are critical studies to determine the efficacy and safety of existing and potential drug compounds. Conventional octadecyl stationary phases for HPLC analysis lack the selectivity to adequately resolve the components of complex pain management assays. The Ascentis Express Biphenyl is an alternative reversed-phase HPLC stationary phase chemistry with unique separation character providing retention and selectivity that are ideal for rapid, efficient drug and metabolite analysis using conditions that are compatible with MS detection.
Fused-Core® Technology
The Ascentis Express Biphenyl is the newest stationary phase available in the Ascentis Express Fused-Core line of U/HPLC columns. The extended aromatic system of the biphenyl moiety yields a more polar stationary phase, increasing retention for compounds that are challenging to analyse using standard C18 chemistry. The hydrophobic, aromatic, and steric properties are inherently orthogonal to alkyl bonded phase columns, providing unique selectivity. Narrow particle size distribution exhibited by the Fused-Core particles yields a more consistently packed bed while the thin, porous shell shortens the diffusion pathway of analytes, resulting in better mass transfer at high flow rates. The enhanced separation kinetics provides superior performance with high sample throughput.
LC/MS Analysis of Pain Management Opioids
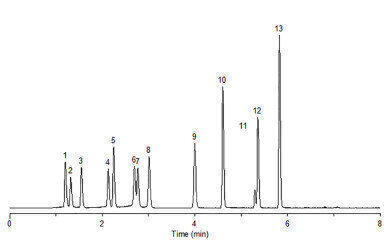
The analysis of pain management opioids requires chromatographic resolution of key isobaric pairs that can’t be determined from MS detection alone. Ascentis Express Biphenyl successfully resolves all critical pairs in the opioid pain panel
Please click here for additional information and product availability.
Figure 1. LC/MS of Pain Management Opioids on Ascentis Express Biphenyl
| Column | Ascentis Express Biphenyl, 10 cm x 2.1 mm I.D., 2.7 µm (64065-U) |
| Mobile phase |
[A] water with 0.1% formic acid [B] acetonitrile with 0.1% formic acid |
| Gradient | 10 to 20% B in 3 min; to 100% B in 3.5 min |
| Flow rate | 0.3 mL/min |
| Column temp | 30°C |
| Detector | MS-TOF, ESI+, XIC |
| Injection | 2 µL |
| Peak | Compound | m/z |
| 1 | Morphine | 286 |
| 2 | Oxymorphone | 302 |
| 3 | Hydromorphone | 286 |
| 4 | Naloxone | 328 |
| 5 | Codeine | 300 |
| 6 | Naltrexone | 342 |
| 7 | Oxycodone | 316 |
| 8 | Hydrocodone | 300 |
| 9 | cis-Tramadol HCl | 264 |
| 10 | Meperidine | 248 |
| 11 | Fentanyl | 337 |
| 12 | Bupprenorphine | 468 |
| 13 | (+/-)-Methadone | 310 |
Events
May 18 2025 Tempe. AZ, USA
May 21 2025 Birmingham, UK
Jun 01 2025 Baltimore, MD, USA
Jun 15 2025 Bruges, Belgium
Jul 14 2025 Kuala Lumpur, Malaylsia