Bioanalytical
SEC analysis of Bevacizumab (Avastin®) and its aggregates and fragments
Jun 24 2020
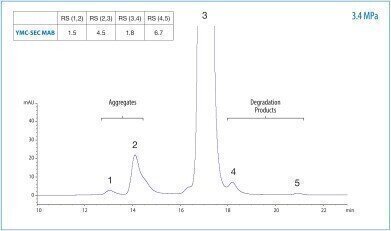
Due to the sizes of monoclonal antibodies (MAbs, about 150 kDa), size exclusion chromatography (SEC) is a standard technique for analysing MAbs such as Bevacizumab (Avastin®). It is also a standard separation mode used in quality control to obtain information about aggregation and/or fragmentation of the MAbs.
In this application note YMC’s dedicated SEC column for antibodies, YMC-SEC MAB, is used to separate the monomer antibody from its aggregates as well as from degradation products in a single run. A neutral phosphate buffer containing 0.2 M NaCl is used as eluent at a flow rate of 0.165 mL/min. UV detection is carried out at 280 nm. The resulting separation provides high resolutions for both the high molecular weight range as well as for the low molecular weight range. The resulting backpressure is only 34 bar/3.4 MPa, while the maximum pressure rating for the column is 140 bar/14 MPa.
YMC-SEC MAB is a column specially designed for the simultaneous analysis of antibodies, their fragments or aggregates. The column is a silica-based size exclusion chromatography column utilising a dihydroxypropyl stationary phase bonded to a silica gel base, specifically designed for antibody separations.
This material offers a 250 Å pore size in 3 µm particle size, making it an excellent choice for separating aggregates from monomer, as well as effectively resolving lower molecular weight fragments. YMC-SEC MAB exhibits excellent resolution and peak shape over a wide molecular weight range of 10 kDa to 700 kDa. Of course, YMC-SEC MAB ensures excellent lot-to-lot reproducibility, which is ideal for quality control.
Events
Apr 22 2025 Kintex, South Korea
Analytica Anacon India & IndiaLabExpo
Apr 23 2025 Mumbai, India
Apr 27 2025 Portland, OR, USA
May 11 2025 Vienna, Austria
May 18 2025 Tempe. AZ, USA