Bioanalytical
New Technical Note: A study of the resolving power of non-porous HIC columns for intact protein analysis
Oct 26 2021
Hydrophobic interaction chromatography (HIC) has regained attention due to its non-denaturing conditions making it a first choice for protein analysis. HIC usually uses a weak hydrophobic stationary phase, such as YMC's BioPro HIC phases. The short chained modified resin interacts with hydrophobic moieties of the protein. The interaction between resin and protein is instigated by a high initial salt concentration.
An inverse salt gradient ensures that the hydrophobic interactions decrease with time, resulting in the elution of the protein. This explains the alternative name of salting-out chromatography.
Comprehensive investigation of HIC separation performance for intact proteins
The Technical Note is based on the work by Prof. Eeltink’s group from the University of Brussels (VUB) on the investigation of separation performance of HIC columns for intact protein analysis. Since the influencing parameters have not been addressed in depth for HIC mode yet, they were the focus in this study:
- 1. Influence of salt concentration
- 2. Temperature impact on retention factor
- 3. Flow rate and gradient time effect on separation performance
- 4. Column length and particle diameter
Use of 2 different YMC HIC columns
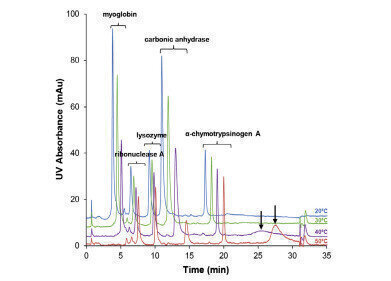
YMC’s BioPro HIC BF and BioPro HIC HT were used in order to determine the effect on intact protein retention. The proteins used for evaluation were cytochrome c, myoglobin, ribonuclease A, lysozyme, carbonic anhydrase, trypsinogen and α-chymotrypsinogen A in varying combinations.
Parameters influencing resolving power
This study by Ewonde et al. provides an overview of how the many parameters can influence the resolving power for intact protein analysis by HIC:
- Increasing the salt concentration and temperature leads to an increase in retention
- Salt concentration has a higher impact than temperature
- Flow rate and gradient time affect each other
- Short gradients benefit from high flow rates and steep gradients
- Increase in column length is beneficial for peak capacity, but requires longer analysis times
- Use of smaller particles allows for increasing peak capacities with shorter analysis times
For detailed information, please refer to the Technical Note based on the original publication or to the study itself.
Events
Apr 22 2025 Kintex, South Korea
Analytica Anacon India & IndiaLabExpo
Apr 23 2025 Mumbai, India
Apr 27 2025 Portland, OR, USA
May 11 2025 Vienna, Austria
May 18 2025 Tempe. AZ, USA