Bioanalytical
How to optimise your oligonucleotide analysis
Aug 24 2021
Therapeutic oligonucleotides with different modifications:
Nucleic acid therapeutics such as silencing RNA duplexes (siRNA), messenger RNA (mRNA) and antisense oligonucleotides (ASOs) are extremely promising candidates for drug therapy in a wide range of diseases. Therapeutic oligonucleotides are often modified to enhance their stability or improve pharmacokinetics. They can be classified by modifications of the backbone, base or sugar moieties and conjugation of the structure [1]. In general, oligonucleotides have phosphodiester linkages (PO). One of the most common modifications contains the replacement of the oxygen atom by sulphur which leads to a phosphorothioate linkage (PS). This PS linkage results in an increase in stability towards nucleases and a prevention of nucleolytic degradation. PS oligonucleotides are often used for in vivo and in vitro techniques as they are more robust than PO.
An additional modification that is often made is a replacement of the nucleophilic hydroxyl moiety at the 2'-ribose position with an O-methyl (2'-OMe) group. This further increases the stability towards nuclease digestion.
AEX and IP-RP as typical separation modes
Their monitoring during purification and in quality control analyses is crucial since impurities and metabolic products can affect their efficacy. Anion exchange (AEX) and ion-pairing reversed phase (IP-RP) chromatography enable the detection of small structural differences. Therefore, they are often used in oligonucleotide analysis.
Several aspects to consider in oligonucleotide analysis
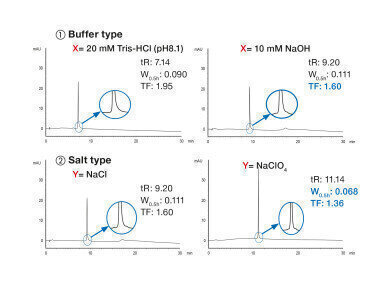
Oligonucleotide LC-analysis is a complex field of interest. There are many factors that contribute to separation and consequently resolution. Due to the retention mechanism of anion AEX, the ionic strength of the eluent is the decisive factor. The type of salt, the initial concentration and the nature of buffer influence the chromatographic behaviour. Almost similar parameters influence IP-RP. Here, the nature and concentration of buffer are important factors. However, chemical modification of the stationary phase, column hardware and temperature provide an even greater impact on chromatographic results.
A new technical note highlights the most important aspects of IEX- and IP-RP method development for the separation of oligonucleotides with slightly differing structures. Since the two modifications described above occur very frequently, they will be the focus of this note.
[1] A. Goyon et al., Characterization of therapeutic oligonucleotides by liquid chromatography, J. Pharm. Biomed. Anal., 2020 April 15, 182:113105 doi: 10.1016/j.jpba.2020.113105.
For further information contact us here.
Events
Apr 22 2025 Kintex, South Korea
Analytica Anacon India & IndiaLabExpo
Apr 23 2025 Mumbai, India
Apr 27 2025 Portland, OR, USA
May 11 2025 Vienna, Austria
May 18 2025 Tempe. AZ, USA