Bioanalytical
High throughput DAR determination of brentuximab vedotin
May 27 2020
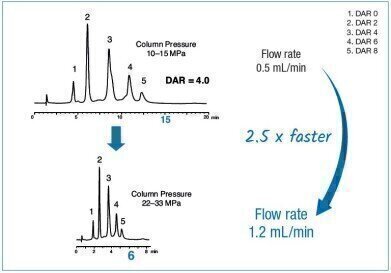
Drug-to-antibody ratios (DAR) of antibody-drug-conjugates (ADCs) such as brentuximab vedotin is important for their therapeutic efficacy and pharmacokinetics. Therefore, control of DAR is a key factor for ADC quality control. Given that, it is important for QC purposes to obtain a satisfying resolution of all DARs and the resulting average ratio whilst performing the separation in the shortest time.
Brentuximab vedotin (Adcetris®) is a cysteine conjugated ADC targeting different types of lymphoma with an average DAR of 4. Typically, this 2nd generation ADC is analysed by hydrophobic interaction chromatography (HIC).
In this application note YMC’s latest hydrophobic interaction chromatography column, BioPro HIC HT, was used. A mobile phase of sodium phosphate buffer at neutral pH with decreasing gradients of the lyotropic salt ammonium sulphate was used. The rigid 2.3 μm non-porous polymer particles are pressure tolerant up to 400 bar and allow rapid analyses through increased flow rates without loss of resolution. This allows flow rates to be increased by 2.5 times with savings of about 60% in time.
Events
Apr 22 2025 Kintex, South Korea
Analytica Anacon India & IndiaLabExpo
Apr 23 2025 Mumbai, India
Apr 27 2025 Portland, OR, USA
May 11 2025 Vienna, Austria
May 18 2025 Tempe. AZ, USA