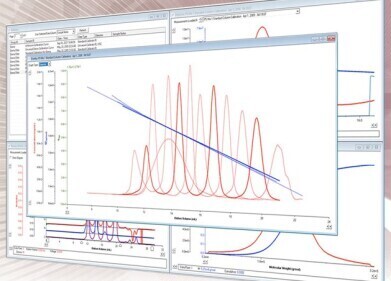
Size exclusion, gel permeation chromatography (GPC)
How Does Liquid Chromatography Work?
May 18 2022
Liquid chromatography is a popular technique for separating complex mixtures in a variety of applications. But how exactly does it work? In this post, we’ll make things a little clearer with an explanation of the principles of liquids chromatography.
Liquid chromatography: the basics
Liquid chromatography starts with a sample mixture, which is carried by the mobile phase. This is loaded onto the stationary phase – a column filled with porous spherical or irregularly shaped particles. The mobile phase moves through the stationary phase – either pulled through by gravity or forced through by high pressure.
Different components within the mobile phase (including the sample mixture) will interact with the stationary phase in different ways. As a result, they’ll pass through at different speeds – known as the retention time. These different retention times can then be used to identify separate components.
The results of liquid chromatography are displayed on a liquid chromatogram. This
More on the mobile phase
The mobile phase is an eluent used to carry the sample through the liquid chromatography apparatus. But it can’t just be any old liquid. A suitable mobile phase needs to be chosen based on its polarity relative to the stationary phase and the sample in question.
Using a polar solvent with a strong polar adsorbent stationary phase, for example, will result in molecules in the sample being displaced and eluted too quickly. The result is little separation, which is hardly helpful when that’s the main goal. It’s typically best to start elution with a lower-polarity solvent which elutes components that are weakly adsorbed to the stationary phase.
What about the stationary phase?
When it comes to the stationary phase, the right adsorbent material needs to be used to separate components effectively. There are a range of options which can be chosen based on both activity and particle size.
Activity refers to how the adsorbent attracts solutes from the sample in the mobile phase. Adsorbents with no water (anhydrous) have the highest activity grade, meaning they attract solutes quicker than the alternatives.
Two of the most popular adsorbents for the stationary phase are alumina and silica gel. That said, alumina is a polar adsorbent, which gives separations by polar interactions. Silica gel is less polar, making it more useful as an all-rounder.
Find out more about liquid chromatography
There is far more to liquid chromatography than meets the eye. Experiments can vary massively depending on the sample in question and, in turn, the different mobile and stationary phases used.
The article, ‘Faster Time to Results for Ultra-Performance Liquid Chromatographic Separations of Metal-Sensitive Analytes’, explores how analytical speed can be improved, specifically when separating metal-sensitive analytes.
Events
May 11 2025 Vienna, Austria
May 18 2025 Tempe. AZ, USA
May 21 2025 Birmingham, UK
Jun 01 2025 Baltimore, MD, USA
Jun 15 2025 Bruges, Belgium