Purification
What Role Does Chromatography Play in Vaccine Production?
Feb 08 2021
The battle against the Covid-19 disease and SARS-CoV-2 virus has entered a new stage now. After isolation and stay-at-home, lockdowns, and test and trace we are now in a stage where governments and health services around the globe are trying to vaccinate as many people as possible as quickly as possible. The pharmaceutical companies and regulators have developed, tested, and manufactured coronavirus vaccines within 12 months of the virus being discovered in Wuhan, China.
It is a massive achievement which could help the world to regain some control of the virus and manage the future spread and outbreaks of Covid-19. There are potentially many different vaccine technologies that are being used in various parts of the world. Most of the production and development details are closely guarded trade secrets. Let’s take a brief tour around the vaccines and see how chromatography is helping to keep Covid-19 at bay.
DNA and mRNA of vaccines
Two of the main vaccines that are mentioned and used in the UK now are the Pfizer-BioNTech vaccine and the Oxford-AstraZeneca vaccine. Both vaccines work by producing replicas of the viruses now famous spikes. But they do it by different methods albeit both using genetic information – DNA in the case of Oxford-AstraZeneca and mRNA for Pfizer-BioNTech.
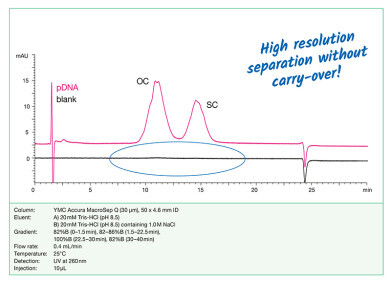
The Oxford/AstraZeneca vaccine uses a ring of double stranded DNA to carry the blueprint of SARS-CoV-2 spike proteins. The team who developed the vaccine enclosed it in an adenovirus, a type of virus associated with the common cold but which in this case doesn’t make us sick. The advantage of using double-stranded DNA in an adenovirus is the stability when compared with mRNA-based vaccines.
Purifying the mix
The Pfizer/BioNTech vaccine is based on messenger-RNA, a simple strand of genetic material that is enclosed in a fat bubble which is a fat-rich cover known as a lipid monoparticle. Unfortunately, the m-RNA is delicate, hence the fat sheath and the requirement to store the vaccine at a low temperature that could make storage and distribution more a problem. Both vaccines work with the genetic material entering host cells and forcing the cells to make spike proteins and spikes. These spikes show on the surface of the cells and are recognised by the body as foreign bodies, this makes the body make antibodies to fight the spikes and virus.
A key element during the production process involves purifying and filtering the vaccines at various stages. One of the key purification steps involves the use of chromatography columns to separate the vaccine from the other parts of the production soup. The use of chromatography in the production and analysis of adeno-virus proteins is discussed in the article, Ultrahigh Sensitivity Analysis of Adeno-associated Virus (AAV) Capsid Proteins by Sodium Dodecyl Sulphate Capillary Gel Electrophoresis.
Events
Apr 22 2025 Kintex, South Korea
Analytica Anacon India & IndiaLabExpo
Apr 23 2025 Mumbai, India
Apr 27 2025 Portland, OR, USA
May 11 2025 Vienna, Austria
May 18 2025 Tempe. AZ, USA