HPLC, UHPLC
Running HPLC with hydrophobic stationary phase and aqueous mobile phase
Sep 22 2020
An octadecyl silane (C18) is probably the most commonly used surface modification in HPLC. It is most stable and highly hydrophobic. The impact of the hydrophobic interaction with the stationary phase can be finely tuned by the organic composition of the mobile phase in reversed phase chromatography. In many separation cases, this is a very efficient way to take mixed substances apart.
Many substances, like peptides and smaller proteins, have advanced tertiary structures maintained by relatively weak non-covalent internal binding interactions. High concentrations of organic solvents, as can happen in RP HPLC, may interfere with these interactions and potentially impair the protein biological activity. Very often, this is a reversible process, but an additional step to restore the structure may be needed. To avoid that, it may be of interest to be able to perform the purification in near or fully aqueous conditions.
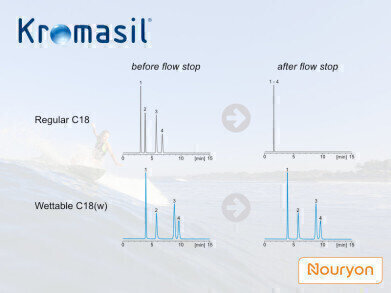
Recently, Kromasil introduced a wettable C18 phase for both production-scale purification of polar substances like biomolecules under aqueous conditions and analytical HPLC applications. Nominally, the wettable phase is still a C18 phase. How can such a phase be “wettable”?
On a regular C18 phase, if 100% aqueous conditions are gradually applied, the mobile phase can keep fairly good contact with the stationary phase as long as the high pressure in the system is maintained. In this situation the pressure forces the mobile phase to be present in the stationary phase’s pores, despite the repealing force from the hydrophobic surface. If the pressure drops (i.e. at flow decrease or stop), the liquid is expulsed from the pores, dramatically reducing the interaction surface between stationary and mobile phases. For the chromatographic properties that means a severe loss in selectivity and coelution of sample components. The surface availability can be restored in the column by switching back to normal phase conditions, though, but the actual chromatographic run can be considered as ruined.
To make the stationary phase wettable in fully aqueous conditions (avoiding dewetting as above), a polar component is introduced on the surface, like a mixed-mode phase. Some have added the polar groups at the root of the surface modifier. The Kromasil wettable phase has it as polar-embedded end-capping. This also allows for a better chemical stability of the phase under acidic conditions compared to other related purification phases on the market.
For more details about the chromatographic conditions for the illustration of this article, as well as learn more about this phase: properties according to the Tanaka test set, examples and current availability, please visit the wettable phase page on the Kromasil website.
Events
Apr 22 2025 Kintex, South Korea
Analytica Anacon India & IndiaLabExpo
Apr 23 2025 Mumbai, India
Apr 27 2025 Portland, OR, USA
May 11 2025 Vienna, Austria
May 18 2025 Tempe. AZ, USA